Kemi
Reaktioner
Hej alle, jeg sidder med en opgave jeg ikke kan forstå - nogen der kan help ? Consider the cell (Fe(s) lFeSO4(aq, a=0,0250)l lHg2SO4(s)l Hg(l). a)Write the cell reaction. b)Calculate the cell potential, the equilibrium constant for the cell reaction, and DeltaG at 25C?
Svar #1
02. januar 2015 af Heptan
Fe (s) | FeSO4 (aq), a = 0,0250 || Hg2SO4 (s) | Hg (l)
Iron is being oxidized by mercury(I)sulphate. Thus the cell reaction
Fe (s) + Hg2SO4 (s) → FeSO4 (aq) + Hg (l)
The cell reaction can be thought of as being a combination of the two half reactions with their respective reduction potentials,
Fe (s) → Fe2+ (aq) + 2 e- Elo = - 0,447 V
Hg2SO4 (s) + 2 e- → 2 Hg (l) + SO42- (slv) Ero = 0,796 V
(sulphate being a spectator ion).
Hence the standard cell potential
Eocell = Ero - Elo = + 1,243 V
and Gibbs free energy
ΔGo = -zFEocell = - 239,9 kJ/mol
and the equilibrium constant
Ko = exp(-ΔGo/RT) = 1,07 * 1042
The equilibrium constant is very large, as we would expect for a reaction between a metal being far to the left in the electrochemical and a metal ion being far to the right.
Svar #2
02. januar 2015 af Heptan
However, the reaction is not under standard conditions, and we must take the reaction quotient into account. The solids and the liquid mercury can all assumed to have an activity close to one, and we need to worry only about iron(II)sulphate. Thus, the reaction quotient is given by
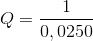
and the cell potential of the reaction can be calculated using Nerns't equation:
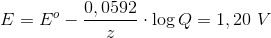
Skriv et svar til: Reaktioner
Du skal være logget ind, for at skrive et svar til dette spørgsmål. Klik her for at logge ind.
Har du ikke en bruger på Studieportalen.dk?
Klik her for at oprette en bruger.







